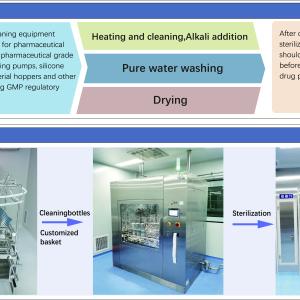
The Industrial GMP Washer Dryer: Revolutionizing Sterile Processing and Workflows
In the intricate ecosystem of pharmaceutical manufacturing, the transition from a wet, cleaned component to a dry, sterile one is a phase fraught with challenges. Moisture is a natural enemy of sterility; it provides a breeding ground for bacteria and can compromise the integrity of subsequent sterilization processes like autoclaving. This is where the Industrial GMP washer Dryer comes into play. Far more advanced than standard laboratory glassware washers, these robust industrial systems are designed to handle high-volume, heavy-duty loads while adhering to the strict hygienic standards of Good Manufacturing Practice (GMP).
The “Industrial” designation implies a capacity for scale and durability that exceeds standard requirements. While smaller benchtop units might suffice for research labs, industrial manufacturing lines require machines capable of processing hundreds of vials, large stainless steel drums, or long transfer piping in a single cycle. An Industrial GMP Washer Dryer is built to withstand continuous operation, often running multiple cycles per day, 365 days a year. The chassis is reinforced to accommodate heavy loads, and the pumping systems are powerful enough to drive water and detergents through complex apparatus with high resistance, ensuring thorough cleaning even in the narrowest lumen of a processing vessel.
However, it is the “Dryer” component that distinguishes this machinery and elevates its necessity. In a traditional washer, items emerge wet and must be manually dried or transferred to a separate drying oven, which increases the risk of contamination. The Industrial GMP Washer Dryer integrates the drying phase seamlessly into the cycle. This is typically achieved through one of two advanced methods: high-velocity HEPA-filtered hot air drying or vacuum drying. In hot air drying, the machine circulates air that has passed through High-Efficiency Particulate Air filters, ensuring that no airborne contaminants are blown onto the sterile surfaces. The high velocity of the air shears water droplets off surfaces, speeding up evaporation. Vacuum drying, on the other hand, lowers the pressure inside the chamber, allowing water to boil off at lower temperatures. This is ideal for heat-sensitive plastic components or complex loads where trapped water might otherwise remain.
The integration of washing and drying into a single automated enclosure offers profound benefits for operational efficiency. In a GMP environment, the fewer open transfers there are, the lower the risk of contamination. By removing the step where an operator must move wet baskets to a separate oven, the closed system is preserved. This is a critical requirement for Automated End-Process Reprocessing (AEPR). Furthermore, modern industrial washer dryers are often designed as pass-through units as well, reinforcing the separation between the “dirty” side and the “clean” side, ensuring that the drying and cooling phase happens under positive pressure in a cleanroom environment.
From a control and compliance perspective, the Industrial GMP Washer Dryer represents the intersection of mechanical engineering and digital intelligence. Contemporary units are controlled via sophisticated HMIs (Human Machine Interfaces) that allow for the storage of hundreds of custom recipes. A manufacturer might have one recipe for stainless steel bioreactor parts (which requires high caustic concentrations and high temperatures) and another for delicate silicone tubing (which requires milder detergents and lower temps). The system automatically adjusts the detergent dosing pumps, water inlet valves, and heating elements to execute these recipes precisely. Moreover, these machines are often compliant with 21 CFR Part 11 regulations, meaning that all digital records of the wash cycles are secure, tamper-proof, and audit-ready.
Sustainability is another emerging focus in the design of these industrial machines. With the pharmaceutical industry under pressure to reduce its environmental footprint, modern washer dryers incorporate energy recovery systems and water recycling technologies. Heat exchangers capture the thermal energy from the hot wastewater to pre-heat the incoming fresh water, significantly reducing utility costs. Similarly, final rinse water, often of high purity (WFI), may be recirculated for use in pre-washes of subsequent loads, provided the system’s conductivity sensors verify the integrity of the water.
In summary, the Industrial GMP Washer Dryer is a sophisticated asset that addresses the twin challenges of hygiene and throughput. By ensuring that equipment is not only chemically clean but physically dry and particle-free, it safeguards the downstream manufacturing process. Its ability to automate a critical, labor-intensive task while providing a digital audit trail makes it indispensable for modern pharmaceutical production. As the industry moves toward Industry 4.0 and smart manufacturing, these machines will continue to evolve, integrating more predictive maintenance capabilities and smarter resource management, further solidifying their role as the workhorses of the sterile manufacturing world.